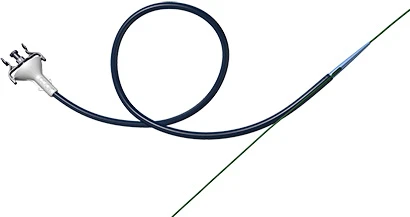
The Food and Drug Administration (FDA) approved Cook Medical's ureteral access sheath, featuring two options for placement. This approval now makes the product available in the United States. The Flexor Parallel Rapid Release Ureteral Access Sheath allows urologists to use a single wire guide that functions as both a working wire and a safety wire.
To place a ureteral access sheath, physicians will often use a working wire guide and a safety wire guide to allow continued access to the kidney. With the Flexor Parallel, physicians can place just one wire that guides the dilator and acts as a safety wire.
The Flexor Parallel has a slit on the dilator that guides the wire outside of the sheath. When the dilator is withdrawn, the sheath and the wire guide remain parallel to each other, which leaves a clear working channel through the sheath.
Physicians can also choose to place the Flexor Parallel in the same manner as a traditional Flexor sheath.
“The Flexor Parallel presents an opportunity to use less product and save costs for hospitals,” states Jean-Marc Creissel, global leader, Cook Medical’s Urology division. “This product means hospitals can stock one sheath that serves two placement preferences.”
Get curated news on YOUR industry.
Enter your email to receive our newsletters.Latest from Today's Medical Developments
- The need for speed in high-speed machining
- FUCHS Lubricants’ NYEMED 7312
- Localizing robotics: Yaskawa's commitment to American manufacturing
- OPEN MIND Technologies’ hyperMILL 2025 CAM software innovations, enhancements
- July Lunch + Learn with SW North America
- Stryker raises full-year guidance despite muted investor reaction
- Unlocking 3D vision: Basler AG launches Stereo ace camera series
- Lisa Anderson, supply chain & manufacturing expert, comments on the One Big Beautiful Act and its implications for US manufacturers





