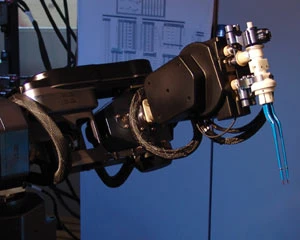
NeuroArm is the first MRI-compatible image-guided surgical robot capable of both microsurgery and stereotaxy. Actuators that utilize revolutionary ceramic motors designed by Nanomotion of the Johnson Medtech network enable the neuroArm to safely operate within an MRI to provide surgeons with unprecedented detail and control.
Working with a team of experts from the University of Calgary and MacDonald, Dettwiler and Associates Ltd. (MDA), Nanomotion collaborated to implement non-magnetic actuators that enable the precision motion necessary for conducting micro-surgical operations safely within the strong magnetic field of an MRI system. In the past, the magnetic nature of electric motors and their metal components restricted surgeons and surgical tools from the MRI environment, making motion impossible.

Non-magnetic actuators enable the neroArm to operate within an MRI.
The neuroArm utilizes 16 of Nanomotion's HR2-1-N-3 piezo ultrasonic nonmagnetic motors, coupled with the company's AB5 drive module. These motors cover six joints, all of which are rotary. Using the real-time visibility into the human body provided by the MRI, the Nanomotion actuators in the neuroArm enable surgeons to manipulate tools at a microscopic scale and conduct surgeries that were previously difficult or impossible.
In addition to the non-magnetic benefit of the ceramic motors, Nanomotion's precision motion control abilities increase the granularity with which a surgeon can work, from within 1/8" using the human hand, to within the width of a hair using the neuroArm. These motors provide surgeons with unprecedented detail and control, enabling them to manipulate tools at a microscopic scale.

Explore the May 2008 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Syringe-less injector system for diagnostic imaging obtains fourth FDA clearance
- Hohenstein Medical debuts enhanced medical device testing capabilities
- Arterex unveils unified brand identity
- Dymax demonstrates light-curing material solutions for medical devices
- Able Medical Devices showcases latest sternal closure solutions
- TMTS 2026 explores AI-powered sustainable manufacturing and more
- QT9 QMS platform streamlines quality management, compliance for medical device manufacturers
- Spineology releases patient-specific expandable spinal implant





