Medical device manufacturers today are faced with increasingly complex regulatory requirements. This increases the costs and risks of product development, and compromises the return on investment. This is partly due to the fact that the work carried out must be carefully and extensively documented, and is also due to the high probability that the product test will fail. To remain competitive, it is essential to have a faster innovation process with short design and validation cycles. The solution is an integrated product development platform for design and analysis as well as for product data management (PDM). The SolidWorks Premium 3D system provides manufacturers with a complete, user-friendly and easy-to-manage package that combines the basic tools for product definition, collaboration, production and documentation.
The SolidWorks software enables easy creation of a parametric, feature-based model of a design concept using solid modeling functions, such as moldings, variable radius fillets, partitions or even draft angles. They define the geometry, function and manufacturability of complex medical devices.
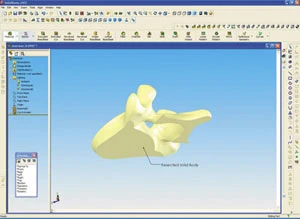
New designs are mainly modifications of already existing products for which 3D models do not yet exist. In this case, ScanTo3D is an essential tool for medical design engineers, who record physical models, existing OEM parts or anatomical objects and have to use the scanned data to create SolidWorks models. Due to the clear 3D visualization, it is possible to spot and eliminate potential problems early on in the development stage.
A medical device is largely made up of bought-in parts, such as semiconductors, printed circuit boards, displays, valves and pumps. These components often have to be redesigned in new projects. This is where SolidWorks' 3D Content-Central portal is helpful, as it gives access to ready-made component models via a broad range of standard, vendor-specific and company-specific design libraries.
By using the simulation software integrated in SolidWorks Premium, different design validations can be processed. Thereby, SolidWorks Simulation determines the stress, strain, deformation and displacement of components under actual operating conditions. SolidWorks Flow Simulation maps the flow of liquids and gases around and in a product. It calculates the flow speed, pressure, temperature and other variables. This tool has already been used for evaluating alternative designs for an artificial heart valve in order to determine the flow turbulence, the pressure loss and the general counter pressure in the valve. This is especially important for achieving optimal handling in the case of small medical devices.
Complex electronic devices often have very little space inside them. Using the CircuitWorks function, ECAD files can be integrated in 3D models and 2D sketches, quickly showing whether the required distance from the metal contacts is maintained when inserting a printed circuit board in the 3D model.
Once the design is optimized, the 3D assembly model can be used to generate 2D engineering drawings. The software automatically creates the drawing view. To ensure consistent documentation and a high degree of control, Solid- Works Workgroup PDM takes care of the product data management, which automatically traces and manages the different versions created in the individual process steps.
Medical device manufacturers today are in fierce competition with one another and face far-reaching regulatory requirements. SolidWorks Premium offers a complete package of solid and surface modeling functions for the design of geometrically-complex and optically-pleasing medical devices. Using this package, medical devices can be designed in a comparatively shorter space of time and with far less risk. At the same time, it provides data management and version monitoring functions that are required for documenting the design process from the first to the last step. In this way, medical engineering assemblies satisfy the regulatory requirements.

Explore the November December 2008 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Syringe-less injector system for diagnostic imaging obtains fourth FDA clearance
- Hohenstein Medical debuts enhanced medical device testing capabilities
- Arterex unveils unified brand identity
- Dymax demonstrates light-curing material solutions for medical devices
- Able Medical Devices showcases latest sternal closure solutions
- TMTS 2026 explores AI-powered sustainable manufacturing and more
- QT9 QMS platform streamlines quality management, compliance for medical device manufacturers
- Spineology releases patient-specific expandable spinal implant





