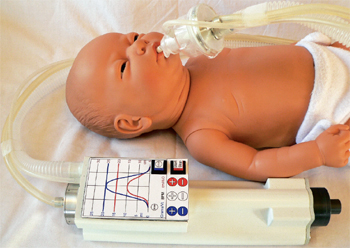
 An early concept design of the Next Step neonatal resuscitator/transport ventilator. Today’s version features a graphical display.More than 100 million babies are born each year, and more than 10 million (10%) require some form of resuscitation assistance. Startling is the fact that more than one million of these newborns die from complications of birth asphyxia.1 An additional two million suffer long-term complications of neonatal Chronic Lung Disease (CLD) – major long-term pulmonary complication of preterm birth.
An early concept design of the Next Step neonatal resuscitator/transport ventilator. Today’s version features a graphical display.More than 100 million babies are born each year, and more than 10 million (10%) require some form of resuscitation assistance. Startling is the fact that more than one million of these newborns die from complications of birth asphyxia.1 An additional two million suffer long-term complications of neonatal Chronic Lung Disease (CLD) – major long-term pulmonary complication of preterm birth.
Of the more than one million newborn deaths, Volutrauma – excessive delivered volume, and Barotrauma – excessive delivered airway pressure, are responsible for the death of many resuscitated using the current methods.2
Initially considered as the major concern to infant mortality or long-term complications was Barotrauma; however, increasing evidence suggests that Volutrauma, excessive volume – leading to over-expansion, and inadequate volume – leading to under expansion/collapse, are more important aetiologically.3
Used for 50+ Years
As horrifying as these numbers are, even more horrific is the fact that the technology used today is from the 1950s. The significance is that self-inflating bags – also known as Bag Valve Masks, which came into use in the 1950s – have little control of airway pressure or volume delivery. Similarly, Flow-Inflating Bags and T-Piece Resuscitators only control airway pressure. None of these resuscitation devices provides the operator warning if the pressure limiter has been over-ridden, nor do they display the volume that has been delivered.
As KM Medical’s Lead Clinical Consultant, Prof. Paul Mathews explains, “Common issues with the currently used resuscitators are that these devices are highly dependent on the rescuer’s skill, experience, and situational reactivity – their ability to act calmly while controlling their response to the increasing stress of the resuscitation itself. The situational reactivity can lead to more forceful and rapid activation of the resuscitation devices, since these are manually operated. This, in turn, can result in the chance of delivering the deadly or damaging increased volumes and pressures to the infant.”
All of these factors – increased volume, pressure, and rate – can act independently, or in combination, to increase the likelihood of volutrauma and/or barotrauma to the patient.
Technology Answers
Clearly, the technology used for neonatal resuscitation needed advancements and improvements, and the KM Medical Team answered that need. Through research, design, and the incorporation of new generation precision, miniature linear servomotors, their game-changing technology not only addresses neonatal use but also adult, military, and home care resuscitation. In addition it delivers a super-light, space-saving transport ventilation application.
Controlling tidal volume, airway pressure, and respiratory rate is really the mantra that resulted in the development of Next Step Neonatal Resuscitator/Transport Ventilator. The staff at KM Medical knew the design needed to address these issues.
“Our overall goal was to control the tidal volume, airway pressure, and respiratory rate – simultaneously – during neonatal resuscitation and transport ventilation in order to reduce the unacceptably high incidence of neonatal death and CLD, as associated with volutrauma and barotrauma,” Mathews says. “Additionally, we wanted to provide a solution in a compact, easy-to-use, lightweight, automated, and low cost-per-use device.”
 Pre-determined program files use the patient’s weight as a key for determining the tidal volume, airway pressure, and respiratory rate.Design, and the components used in the Next Step, is what makes this a game-changing technology from the current resuscitation options, while delivering safer outcomes.
Pre-determined program files use the patient’s weight as a key for determining the tidal volume, airway pressure, and respiratory rate.Design, and the components used in the Next Step, is what makes this a game-changing technology from the current resuscitation options, while delivering safer outcomes.
The Next Step consists of a high-precision miniature linear servomotor connected to a piston, which mounts to a cylinder to act as a linear pump. Induction valves are located at one end of the cylinder while at the other end of the cylinder is a bi-directional valve.
As is typical with ventilation devices, this bi-directional valve directs the flow of air or an air/oxygen mixture from the resuscitator to the patient, as well as the flow of exhaled air from the patient out through an exhaust port. The bi-directional valve connects to either a mask or an endotracheal tube.
As an electronically driven resuscitator/ventilator, the Next Step incorporates a micro-controller for precise control of the linear servomotor. The servomotor is connected to a piston that provides the accurate tidal volume, airway pressure, and respiratory rate – parts that are missing from currently used Flow-Inflating Bags, Bag Valve Masks, and T-Piece Resuscitators. This control is what follows best practice guidelines, delivering safety for those requiring resuscitation while reducing the chance for CLD or death.
Management of volume, pressure, and rate are via a CPU. Volume is determined by the distance traveled by the piston; the force of the piston determines pressure; and the number of piston strokes determines rate over time. Delivery of continuous positive airway pressure (C-PAP) is delivered with a miniature blower motor via a patented airway circuit. An off-the-shelf pressure controller, fitted to the exhaust port of the patient valve, controls positive end-expiratory pressure (PEEP) – a term used in mechanical ventilation to denote an airway pressure that is kept above atmospheric pressure at the end of the expiratory cycle.
“A touch sensitive LCD screen or buttons – depending on user-preference – allows both the setting and display of either pre-programmed profiles or operator determined settings,” Mathews explains. “Pre-determined program files use the patient’s weight as a key for determining the tidal volume, airway pressure, and respiratory rate.”
The graphical display can be stored to assist in the setup of other ventilator systems, for subsequent clinical analysis or for teaching purposes.
“This is particularly significant for neonatal applications as there is a growing awareness that resuscitation of neonatal patients using traditional resuscitators providing only pressure control increases the risk of Volutrauma,” Mathews emphasizes. “The algorithm that calculates and applies the patient volume, pressure, and flow characteristics is designed to be patient-specific – allowing the Next Step to initiate ventilator support at a predetermined ideal normal level, which can be modified by the responder as needed.”4
Additionally, the Next Step does not require any external gas source to function – running off its rechargeable internal battery pack for four hours or from any external power source from 12V to 240V. Total weight of the prototype unit, including battery pack is approximately 2 lb (800g), with all patient-contaminated airway components being low-cost, single-use consumables.
Collaborative Work
Four years is what it took – for Next Step to offer the next step in respiratory care. Work on the design of the Next Step was conducted both internally by the KM Team and externally. A contract with UniServices and the University of Auckland, aided development of the Next Step, as well as KM Medical’s intermittent use of Plume Product Design Ltd. during a three-year time frame. Professor Enrico Haemmerle, Advanced Mechatronics Research Group, School of Engineering, The University of Auckland headed the project. As the design is continually evolving in response to clinical and market feedback – as well as from the study of new fields of applications – new prototypes and testing continue to evolve.
Initially developed to specifically overcome the shortcomings of traditional neonatal resuscitation devices, the creators ended up developing a new generation device.
Currently in discussion with potential licensees, KM Medical is also working toward clinical trials and FDA approval. Once in production, the advancements brought to the resuscitation/ventilation market will be that of technology available in the 21st Century, with more lives saved and less chances of CLD.
KM Medical Ltd.
Auckland, New Zealand
kmmedical.co.nz

Explore the April 2011 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Stryker’s flexible syndesmotic fixation device stabilizes ankle injuries
- Mergers & acquisitions news: MGS, Quantum Surgical bolster medtech portfolios
- Exchangeable-head solid carbide cutting tools
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables





