PHOTO COURTESY OF NEXTREMITY
FDA 510(K) clearances
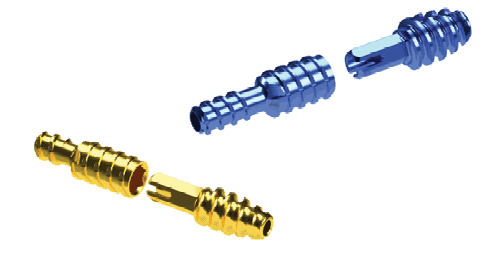
Nextremity Solutions Inc.’s upcoming DuoHex CH Cannulated Hammertoe System has an optimized array of implant threads for a variety of patient anatomy, a fully-guided cannulated system, and in-situ adjustment. DuoHex CH addresses hammertoe deformity in patients and provides surgeons with a two-piece threaded implant for optimum bone purchase. The design of the cannulated implants and instruments provides targeting and technique guidance for repeatable outcomes. Implant-to-implant rotational stability is made possible by the implant’s hexagonal locking mechanism.
Royal Philips Philips Biosensor BX100 is cleared to help hospitals monitor COVID-19 patients. The wireless, wearable biosensor adheres to the chest to collect, store, measure, and transmit respiratory rate and heart rate – the top two predictors of deterioration – every minute, as well as contextual parameters such as posture, activity level, and ambulation. The solution is CE marked and supports surveillance of higher acuity patients moving from intensive care units (ICUs) into lower acuity general care areas. Lightweight and disposable, the 5-day, single-use patch can integrate with a scalable hub to monitor multiple patients across multiple rooms.
STANDARDS
A proposed ASTM Int’l standard could provide functional ergonomic guidelines for exoskeleton design, construction, and use. ASTM’s committee on exoskeletons and exosuits is developing the proposed standard with guidelines to reduce the risk of injuries or illnesses resulting from repeated trauma or repetitive motions associated with exoskeleton use in industrial, military, medical, first responder, and recreational cases.

Explore the August 2020 Issue
Check out more from this issue and find you next story to read.
Latest from Today's Medical Developments
- Tsugami America’s Technical Center in Minnesota
- RobOps Copilot for AI-powered robot optimization
- US companies invest heavily in robots
- #34 Lunch + Learn Podcast - Cobots' potential to revolutionize aerospace manufacturing with Techman Robots
- Universal Robots announces seamless integration with Siemens PLCs
- This month's Manufacturing Lunch + Learn is May 16
- Ultrahuman wearable technology
- Additive Manufacturing for Aircraft Cockpit Interior Components