TRUMPF
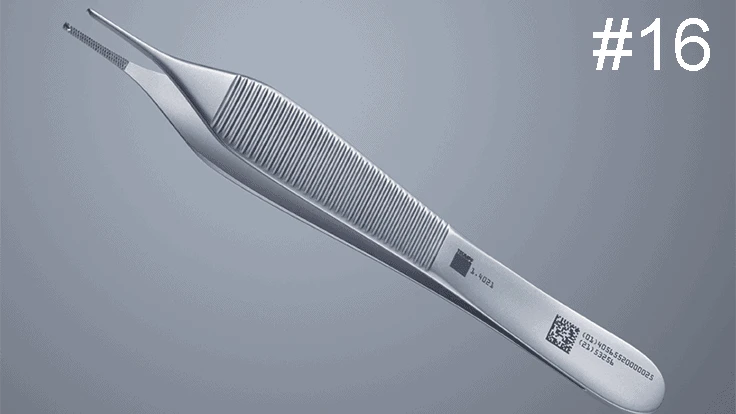
Final versions of the European Union Medical Device Regulation (EU MDR) and the In Vitro Diagnostic Regulation (IVDR) go into effect May 2020 and 2022, respectively. These regulations, alongside the already launched U.S. Food and Drug Administration (FDA) unique device identification (UDI) ruling, have impacted how medtech companies develop and market products for use and distribution. Even with seven notified bodies and counting, manufacturers remain concerned with the looming compliance deadline. A recent statement from the European Commissioner for Health emphasized the May 26, 2020, MDR deadline holds, despite any postponement of the EUDAMED database.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





