CREDIT: ABLE MEDICAL DEVICES
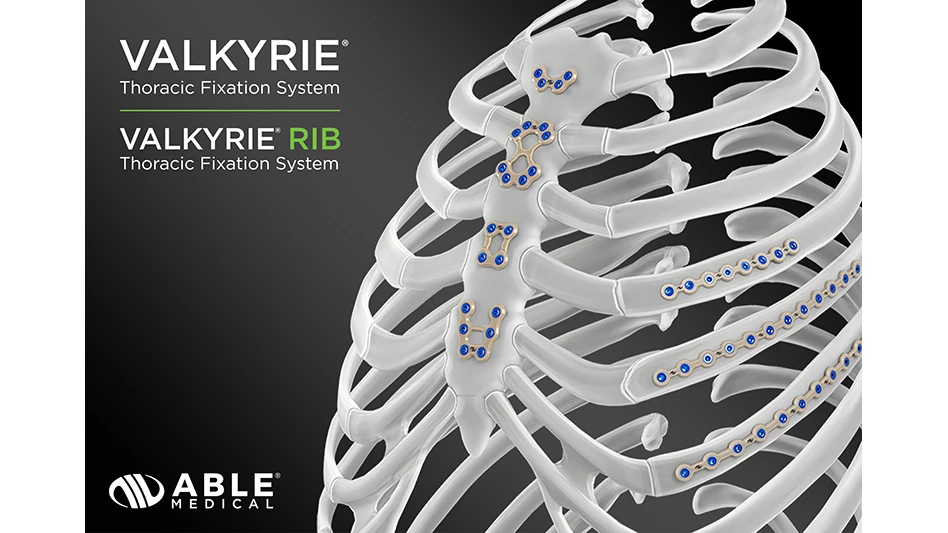
Able Medical Devices, the first company to introduce a PEEK rigid fixation system for sternal closure, will showcase its latest sternal closure solutions at the Society of Thoracic Surgeons (STS) 2026 Annual Meeting in New Orleans, Louisiana. The company’s advanced PEEK-based sternal closure technology reflects its ongoing commitment to improving patient outcomes and surgical efficiency.
Able Medical’s sternal closure system utilizes PEEK (polyether ether ketone) to provide surgeons with a strong, lightweight and biocompatible alternative to traditional metal implants. PEEK’s radiolucency and flexibility make it an ideal material for patients who require stability, comfort, and improved visualization during the healing process.
“We’re excited to be among the first to bring a PEEK sternal closure system to market,” says Peter Didyk, SVP & managing director at Able Medical. “Our team’s collaboration with surgeons and engineers have driven the development of a solution that enhances patient care while simplifying the surgical experience for the cardiothoracic O.R. teams.”
Able Medical will exhibit its PEEK sternal closure products at STS 2026, taking place January 29 to February 1, 2026, in New Orleans, Louisiana. Attendees are invited to visit Able Medical at booth 1009 to learn more about the company’s sternal closure platform, upcoming innovations, and custom design capabilities.
Latest from Today's Medical Developments
- TMTS 2026 explores AI-powered sustainable manufacturing and more
- QT9 QMS platform streamlines quality management, compliance for medical device manufacturers
- Spineology releases patient-specific expandable spinal implant
- Nordson EFD to demonstrate medical assembly automation applications
- Strategic partnership created to support early medical device innovation
- The latest workholding technology to boost productivity and efficiency
- Multi-articulated, body-powered prosthetic hand now available in US
- There’s still time to register for our 2026 Manufacturing Forecast roundtable!





