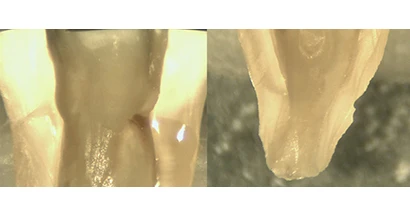
Officials from Sonendo, the company that is developing a revolutionary technology for the endodontic marketplace, announced that the company has received 510(k) FDA clearance for the first generation of its Multisonic Ultracleaning System set to launch in 2014. The device is designed to be a minimally invasive, disruptive technology that uses multiple wavelengths of sound to simultaneously clean the entire root canal system.
"This 510(k) clearance is an important milestone for Sonendo and puts us on the path for a commercial launch in 2014," says Bjarne Bergheim, president and CEO of Sonendo. "Our goal is to transform endodontics by improving the clinical quality and business performance of practices performing root canal therapy."
510(k) clearance is released through the Center for Devices and Radiological Health (CDRH) within the Food and Drug Administration (FDA). The clearance gives Sonendo the right to market the new medical device within the United States.
Sonendo's system is not yet commercially available.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





