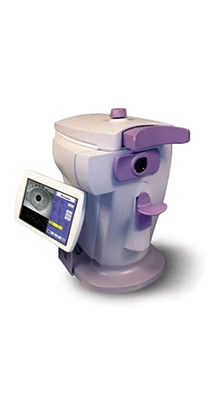
Freedom Meditech Inc. officials have launched the ClearPath DS-120 Lens Fluorescence Biomicroscope. Cleared by the U.S. Food and Drug Administration (FDA) in January 2013, the ClearPath DS-120 is a first-in-class, non-invasive medical device tool designed to quickly and accurately measure autofluorescence through a six second scan of the crystalline lens of the eye. A significant number of independent peer-reviewed studies have suggested that elevated lens autofluorescence may be an early indicator of the presence of diabetes.
Nearly 104.8 million Americans have diabetes or pre-diabetes, costing an estimated $250 billion annually. Complications of diabetes including blindness, kidney failure, and amputations can be avoided with early disease detection and intervention.
"There is a great need for early detection of diabetes beyond the currently available blood tests and doctor visits," said Daniel Einhorn, M.D., an endocrinologist with the Scripps Health System. "The Centers for Disease Control and Prevention have shown that almost 80 million Americans have pre-diabetes, a stage where intervention is most cost-effective. There is increased public awareness of this, and the ophthalmic community could be at the center of one of the most important public health opportunities of our generation."
The ClearPath DS-120 produces a quantitative result that is immediately available to the optometrist and patient, and can be electronically transmitted to a patient or referral healthcare provider. The scan does not require a blood draw or pupil dilation. The device has a small footprint, sits on a tabletop, and employs an easy to use touch screen display that can be wirelessly linked to a practitioner's electronic medical record system.
"With over 100 million eye exams now performed in the U.S. annually, the eye-care provider is a gatekeeper for people within the age range when adult onset diabetes typically presents," explains Craig Misrach, chairman and CEO of Freedom Meditech. "The ClearPath DS-120 has the potential to change the standard optometry visit and increase the role of the optometrist in the management of their patients' overall health."
About ClearPath DS-120
The ClearPath DS-120 Lens Fluorescence Biomicroscope is cleared by FDA as a tool for the measurement of autofluorescence by scanning the crystalline lens of the eye with a blue light. In independent scientific studies published in peer-reviewed journals, elevated autofluorescence measurements have been linked to high levels of advanced glycosylated end products, which accumulate as a result of the aging process, and the presence of systemic disease.
The ClearPath scan is pain free, takes just six seconds, and produces an immediate, quantitative result available to the patent and health care provider. Unlike some eye exams, the scan does not require dilation. The ClearPath is completely non-invasive and does not require a blood draw to produce a result.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





