CREDIT: HOBBS MEDICAL
Hobbs Medical is proud to announce the launch of the CleanCapture Aspiration Catheter at ACG 2025. Dr. Ali Rezaie, MD, MSc, FRCP(C), Medical Director of the Cedars-Sinai GI Motility Program and Lead Developer of the CleanCapture device, and Brian Murray, General Manager at Hobbs Medical, Inc., attended the product debut (pictured).
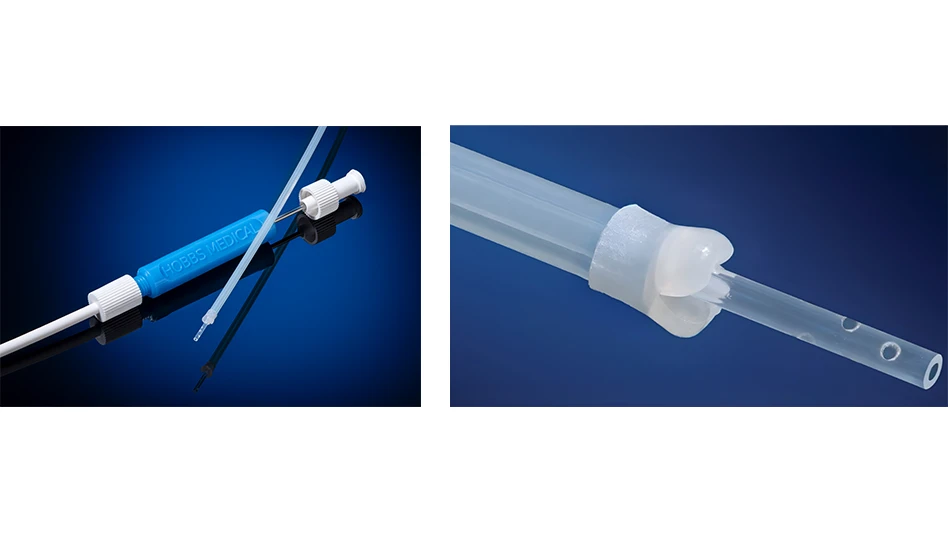
The CleanCapture Aspiration Catheter introduces a novel advancement in microbiome sampling. Engineered with a patent-pending protective membrane at the deployment tip, the device shields the aspiration channel during insertion and withdrawal, reducing contamination, which is a long-standing challenge in small-bowel aspirate collection.
Additional design elements, including a translucent outer sheath and a spiral hole pattern at the distal collection tip, further support efficient fluid retrieval. This first-of-its-kind catheter has the potential to advance diagnostic capabilities in SIBO research and broader GI microbiome analysis.
“The device enables aspiration with minimal contamination. We hope this will optimize endoscopic aspiration for both clinical practices and microbiome research,” says Dr. Rezaie.
“For over four decades, Hobbs Medical has manufactured and supplied quality devices for GI and pulmonary endoscopy procedures. We are grateful for the collaborative innovation that brought the CleanCapture and our other novel devices to market. We are excited for what these technologies will bring to the GI community,” said Murray.
For more information on the CleanCapture Aspiration Catheter, visit the Hobbs Medical Product Page.
Latest from Today's Medical Developments
- Gore completes acquisition of Conformal Medical
- Medical textiles designed for cardiovascular, orthopedic, dental prosthetic applications
- Micro-precision 3D printing: Trends and breakthroughs in medical device manufacturing
- One-component, dual-cure adhesive system for medical device assembly
- #82 Manufacturing Matters - Forecasting 2026 with GIE Media's Manufacturing Group
- Flexing prosthetic finger offers lifelike appearance and movement
- How the fast-evolving defense market impacts suppliers
- Medtronic’s Hugo robotic-assisted surgery system makes US debut





