CREDIT: www.microsystems.uk.com
In the tightly regulated and innovation-driven medical device industry, the seamless transition from concept to scalable manufacturing is critical. By integrating DFMEA, mold manufacture, mold testing and development, validation, metrology, and production molding in a holistic approach to manufacturing medical devices, this strategy accelerates market entry, strengthens design control, and ensures consistent clinical performance in mass production environments.
Why integration matters in the medical device industry
In medical device development, ensuring that molded components meet strict tolerances, biocompatibility standards, and sterilization requirements is critical to patient safety and regulatory approval. Governed by global standards like ISO 13485 and FDA 21 CFR Part 820, the process demands robust design controls, traceability, and validation. However, many medical device projects still operate in isolated stages, separating product design, tooling, validation, and manufacturing, which extends timelines and risks quality and compliance. A holistic development model that integrates mold design, testing, validation, and manufacturing readiness from the outset is increasingly essential for achieving scalable production without compromising safety, efficacy, or regulatory approval.
The importance of early risk assessment through DFMEA
Integrating design failure mode and effects analysis (DFMEA) early in the design-for-manufacturing (DFM) process, particularly at the concept and design phases, lays the groundwork for robust, compliant, and cost-effective manufacturing. DFMEA systematically identifies and evaluates potential failure modes in mold design, such as incomplete filling, flash, or deformation, and prioritizes corrective actions using Risk Priority Numbers (RPN) based on severity, occurrence, and detection. This proactive approach not only reduces the risk of defects and costly rework but also strengthens the foundation for
later validation activities (IQ/OQ/PQ) and regulatory submissions by demonstrating risk control measures were systematically applied from the outset.
By involving cross-functional teams, including tooling engineers, quality, and regulatory stakeholders, during early-stage DFMEA, teams can:
- Anticipate potential failure modes such as dimensional non-conformance, material incompatibility, or deformation during cooling cycles.
- Address mold-related risks, such as flash formation, short shots, or sink marks, which could compromise device performance or create particulate contamination.
- Drive design modifications that optimize flow paths, minimize internal stresses, and eliminate unnecessary features that complicate tooling or validation.
- Improve parting line definition, gating strategies, and ejection systems to ensure clean, repeatable demolding, which is crucial for high-volume components like diagnostic cartridges or fluidic manifolds.
For Simulation-Driven Mold Development, tools such as Moldflow, Moldex3D, and SolidWorks Plastics allow for predictive modelling of complex features, including thin-walled sections found in drug delivery devices or in-vitro diagnostics, hence informing mouldability, sterility assurance and functional performance under clinical use.
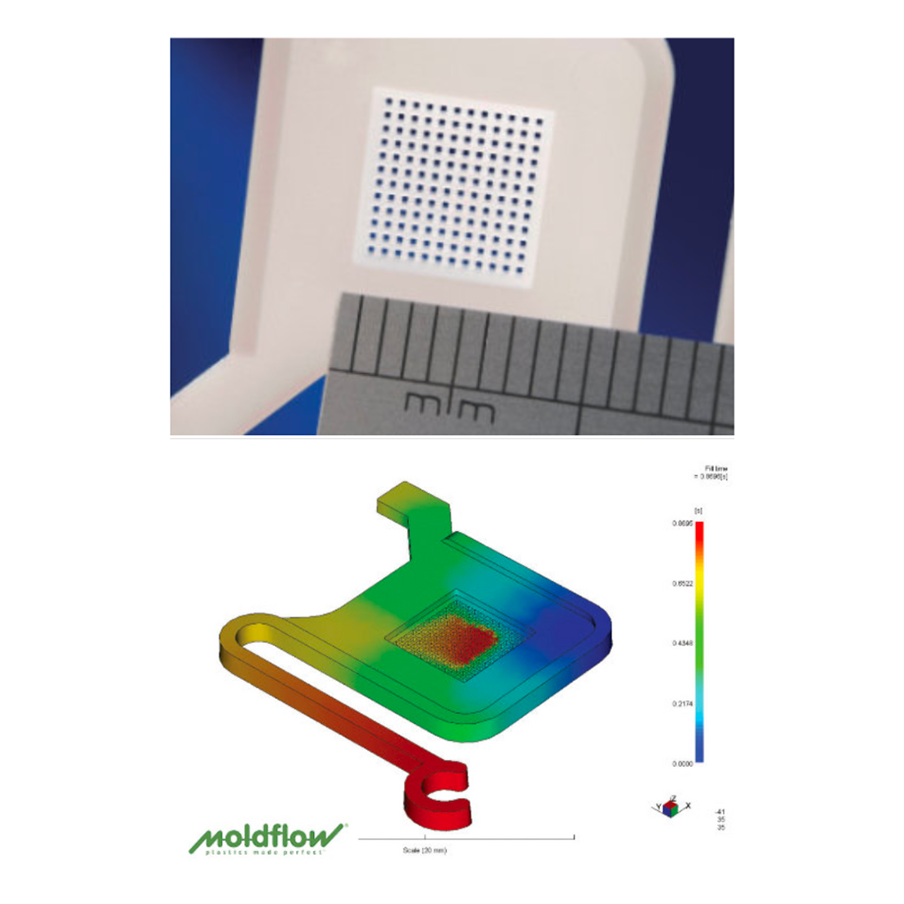
Mold manufacturing: Precision execution that defines success
While design and simulation are foundational, the actual manufacture of the mold tool is the point where theory meets reality. In medical device production, where tolerances are often
within microns, and consistency is tied directly to patient safety, the quality and precision of the mold build are non-negotiable.
The role of expertise and specialized facilities: Medical-grade tooling requires more than just CNC machining. It demands:
- Experienced toolmakers familiar with medical component geometries, high-cavitation tooling, and multi-material interfaces.
- Controlled environments, such as temperature- and humidity-regulated shops to prevent thermal distortion during build/inspection.
- Strict material controls for tool steel and coatings, especially when handling bioresins, implant-grade polymers, or materials requiring validation for cytotoxicity or extractables/leachables.
Precision and repeatability: A single cavity defect, such as core mismatch, improper venting, or gate burr, can result in dimensional out-of-spec parts, sterility breaches due to particulate or flash, or tool wear or failure during high-volume runs. Precision machining techniques utilizing high end equipment for EDM (Electrical Discharge Machining), 5-axis CNC, and polishing to SPI A1 finishes are essential for meeting demanding cosmetic and functional criteria, particularly for optical or wearable medical devices.
Longevity and maintenance: Medical molds are often expected to support millions of shots. Precision mold manufacture ensures initial part quality, reduced maintenance intervals, tool longevity with consistent output across long production campaigns, and minimized downtime due to premature wear or breakdown.

Mold testing and development: De-risking before process validation
Before entering formal validation (IQ/OQ/PQ), a dedicated mold testing and development phase is essential to ensure that the tool performs as intended under real-world process conditions. It provides the bridge between theoretical design and real-world, validated manufacturing, hence protecting both the patient and the program timeline.
T0/T1 trials – First look at part quality: Initial mold sampling (T0 or T1 shots), provides a controlled environment to assess fill patterns, flash, venting, part warpage, and shrinkage using high-resolution metrology. It also allows testing of critical features such as seal integrity, assembly fit, and flow channels. These early trials help identify tool tuning needs (e.g., gate size, vent depth, cooling adjustments) without delaying formal validation.
Process window mapping: Mold testing is used to define preliminary process parameters across a safe and reproducible window. By fine-tuning these variables in controlled, iterative testing, development teams can lock in process windows that support robust OQ/PQ performance later, including fill speed vs. cavity pressure, cooling time vs. dimensional stability, and packing pressure vs. part weight.
Material behavior validation: Especially in devices using engineered or bioresorbable resins (e.g., PEEK, PC, TPU), mold development allows teams to understand shear sensitivity and thermal degradation risks, to confirm sterilization stability post-molding (e.g., gamma, EtO, autoclave), and to verify lot-to-lot consistency for validated polymers.

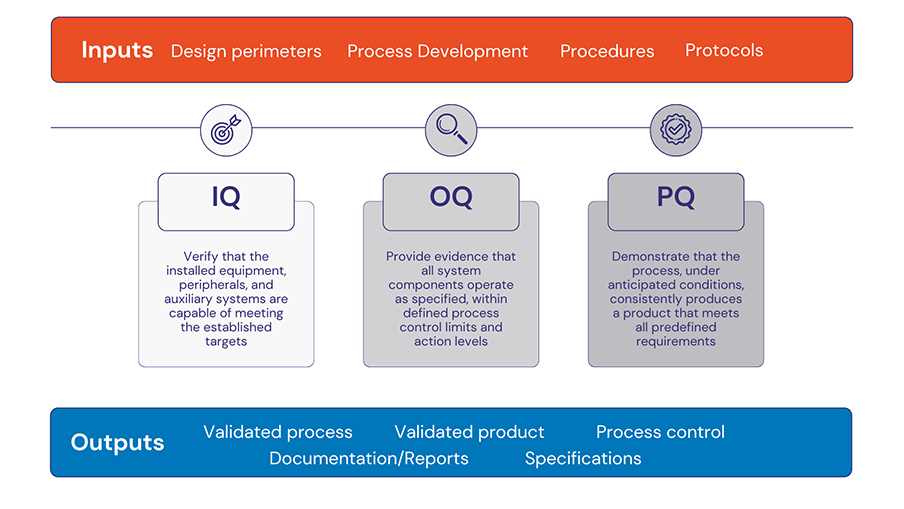
Integrated validation: From prototype to production within regulatory boundaries
IQ/OQ/PQ integration in medical device lifecycle: Validation is a regulatory mandate and a quality imperative. Proper implementation of Validation protocols from Installation Qualification (IQ), Operational Qualification (OQ), to Performance Qualification (PQ) ensures early demonstration of process capability under Good Manufacturing Practices (GMP), documented evidence for submission to FDA (e.g., 510(k), PMA) and Notified Bodies, as well as seamless incorporation into Design History Files (DHF) and Device Master Records (DMR).
Metrology: The backbone of precision and regulatory assurance
In a world where ±10 microns can mean the difference between clinical success and product failure, metrology is the thread that connects design intent, process validation, and production performance. It is indispensable for de-risking development, shortening feedback loops, and demonstrating compliance across all regulatory audits.
Metrology in mold design: During the mold design and initial tool qualification phase, metrology ensures that the tool geometry matches CAD models precisely. Any deviations can affect part tolerances, fit with mating components, or functionality. Using a wide range of metrology equipment from coordinate measuring machines (CMMs), digital microscopes, white-light interferometry, CT Scanning and more, full confidence can be achieved in the steel dimensions and subsequent plastic molding dimensions.
Metrology in production control: In mass production, metrology is essential for:
- Statistical Process Control (SPC): Inline measurement of key features to track Cp/Cpk values and detect drift.
- Automation Feedback: Vision systems and laser profilers feed dimensional data directly to MES/QMS systems, enabling closed-loop corrections.
- Regulatory Documentation: Measurement data supports batch release, device history records (DHRs), and substantiates product claims in 510(k) or CE submissions.
Digital metrology tools, such as CT scanning for internal geometries or digital micrometry integrated with IoT systems, are enabling real-time, high-resolution insight into critical tolerances. This supports predictive maintenance, zero-defect strategies, and continuous validation aligned with FDA’s emerging Computer Software Assurance (CSA) model.

Mass production success: Where integration becomes non-negotiable
Clinical performance depends on manufacturing consistency: In medical device applications, scalability is not simply a volume issue, it is a quality and risk issue. Variations introduced in scale-up (e.g., due to tooling wear, operator variability, or machine changes) can result in device malfunctions or failures in the field, nonconformances that trigger recalls or CAPA investigations, or delayed product launches due to re-validation requirements. An integrated approach ensures that the validated process scales predictably, preserving clinical efficacy and compliance.

Feedback loop from production to design: In a holistic system, production data is continuously fed back into product design and tooling optimization. This loop supports continuous improvement in design for robustness and assembly (especially in combination products), early detection of shifts in Cpk or defect rates, and automated documentation updates for regulatory submissions.
Overall, partnering with a full-service medical device supplier that manages the entire development process on-site, from design and mold engineering to validation and mass production, is critical for ensuring technical precision, regulatory compliance, and clinical reliability. In a field where components must meet strict tolerances, biocompatibility, and sterilization standards, a holistic approach aligns design, manufacturing, and quality from the outset. A single-source partner reduces risks from vendor handoffs, streamlines communication, and accelerates time-to-market. This integration enables earlier design-for-manufacturability input, tighter process control, and smoother validation (IQ/OQ/PQ), ensuring safe, high-quality devices that meet regulatory and clinical demands.
Latest from Today's Medical Developments
- Ilika, Cirtec advance strategic partnership to commercial level
- Engineered fluids offer full lifecycle solutions for medical device development
- Syringe-less injector system for diagnostic imaging obtains fourth FDA clearance
- Hohenstein Medical debuts enhanced medical device testing capabilities
- Arterex unveils unified brand identity
- Dymax demonstrates light-curing material solutions for medical devices
- Able Medical Devices showcases latest sternal closure solutions
- TMTS 2026 explores AI-powered sustainable manufacturing and more