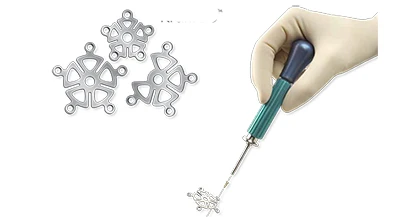
Integra LifeSciences Holdings Corp. officials announce the launch of the Integra Compact Cranial Closure System, which provides titanium implants for non-loadbearing (non-facial) operative cranial neurosurgical procedures. Included with the system is a new Integra Compact Disposable Powered Driver, a pre-sterilized, disposable, battery powered screwdriver that is used for rapid fixation of the implant's plates and screws. Both products have received 510(k) clearance from the United States Food and Drug Administration (FDA).
The Compact Cranial Closure System's benefits include a small and simple tray design, which enhances efficiency in both the operating room and the hospital central sterilization department, and low profile plates, which are thin but strong titanium alloys, and offer excellent patient aesthetics. The new pre-sterilized, disposable, battery powered screwdriver offers neurosurgeons a very convenient single-use device that is available whenever and wherever the surgeon needs it.
"Our new cranial closure system helps round out our neurosurgical product offerings," states Pete Ligotti, Integra's vice president of marketing, neurosurgery. "Cranial closure occurs immediately following repair of the dural lining surrounding the brain. We offer an extensive line of our DuraGen products for dural repair, and now, with our new cranial closure system, we provide neurosurgeons with the full line of products that they need for a cranial closure procedure."
Neurosurgical cranial fixation sets are used in more than 200,000 cranial procedures in the U.S. annually, including treatment of head trauma injuries, biopsies and tumor removal.
Latest from Today's Medical Developments
- Stryker’s flexible syndesmotic fixation device stabilizes ankle injuries
- Mergers & acquisitions news: MGS, Quantum Surgical bolster medtech portfolios
- Exchangeable-head solid carbide cutting tools
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables





