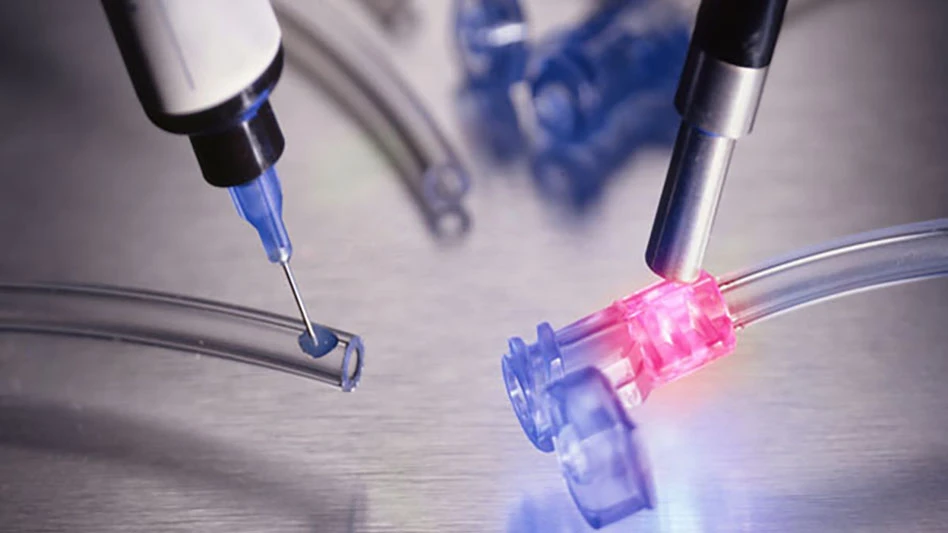
CREDIT: KISTLER GROUP

Kistler Group, developer of dynamic measurement technology, and ATS Life Sciences Systems (LSS), an automation solutions provider, have joined forces for a state-of-the-art medical device assembly line. Combining advanced sensor technology from Kistler with a patented digital motion system from ATS LSS, Symphoni platform delivers real-time in-line quality assurance for medical devices, up to 320 parts per minute with 90% less tooling and full traceability. The platform sets a new benchmark for scalable, data-driven automation in medical device manufacturing, supporting regulatory-compliant production, audit-ready data, and flexible handling of multiple product types on a single line.
For medical device manufacturers, scaling production is not just about building a functional production line but also about making sure that millions of devices are produced flawlessly. Yet, many conventional assembly processes remain a ‘black box,’ with quality assurance limited to pre- and post-assembly inspections. These legacy methods create significant challenges: they consume valuable floor space and require numerous specialized tools. Beyond operational inefficiencies, they affect regulatory compliance, yield, and scalability. To meet stringent standards such as FDA 21 CFR Part 11 and EU MDR, manufacturers must demonstrate consistent product quality providing fully traceable and audit-ready process data. Optimized layouts and minimal downtime are also key factors.
Collaboration drives innovation in high-speed medical device assembly

To address these challenges, ATS LSS and Kistler Group collaborated for an automated assembly line that makes previously invisible aspects of production measurable and controllable. The system integrates ATS LSS’ expertise in digitally synchronous motion and modular automation with real-time force and displacement sensing from Kistler, enabling assembly and in-line verification of medical devices at speeds exceeding 300 parts per minute and the flexibility to handle multiple product types on a single line. These improvements reduce overall cycle time from 3,750ms to 750ms, delivering rapid, precise, and traceable inline process monitoring.
From assumptions to assurance through real-time data
At the same time, the real-time force and displacement monitoring from Kistler allows Symphoni to measure and verify critical steps of medical device assembly as it happens. In-line waveform analysis detects potential failure modes, while a patent-pending inertia compensation system neutralizes the effects of high-speed motion on force measurements. These capabilities enable precise evaluation of force and distance, reducing false positives and negatives and eliminating the need for separate inspection stations. All collected real-time data can be stored across multiple databases, supporting process optimization, regulatory compliance, and rapid ramp-up of new production lines.
Latest from Today's Medical Developments
- Platinum Tooling expands internal coolant tool offering
- US metalworking machinery orders through November 2025 reveal 17.8% increase from first 11 months of 2024
- Precision metering pumps for medical device manufacturing
- #81 Manufacturing Matters - Additive Manufacturing Analysis, Trends, Forecasts with Terry Wohlers
- Velosity opens precision development center to accelerate medical device product launch
- A look at the latest in the defense industry
- EMCO manufacturing showroom offers customers hands-on milling, machining engagement
- Workholding Roundtable to feature expert insights on a booming market