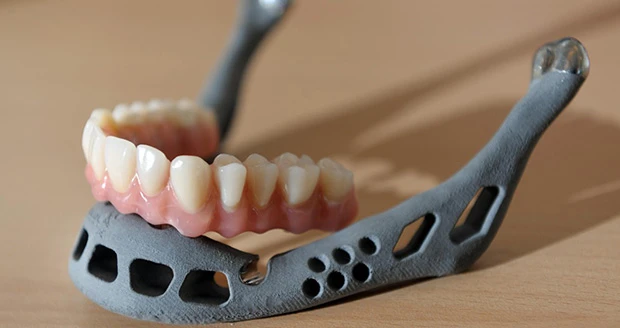
Silver Spring, Maryland - The FDA is holding a public workshop - Additive Manufacturing of Medical Devices: An Interactive Discussion on the Technical Considerations of 3D Printing, on Oct. 8-9, 2014, at the FDA White Oak Campus, 10903 New Hampshire Ave., Bldg. 31, Rm. 1503 (the Great Room), Silver Spring, MD, 20993.
The purpose of this workshop is to provide a forum for FDA, medical device manufactures, additive manufacturing companies, and academia to discuss technical challenges and solutions of 3D printing. The Agency would like input regarding technical assessments that should be considered for additively manufactured devices to provide a transparent evaluation process for future submissions.
Source: FDA
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





