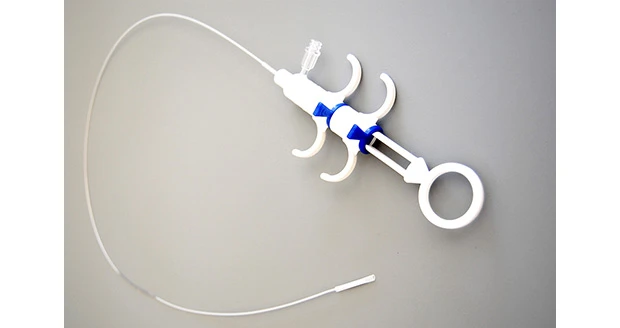
Carlsbad, California – ArtVentive Medical Group Inc. officials announce that the company has received U.S. Food and Drug Administration (FDA) clearance for the Endoluminal Occlusion System (EOS). Designed for use in the peripheral vasculature, the EOS offers immediate, complete, and total occlusion in arterial and venous settings.
“I see the EOS device as a potential game changer for physicians and their patients,” said Dr. Anthony Venbrux, interventional radiologist at The George Washington University Hospital in Washington, D.C. “In my experience with the device, it has shown immediate and complete vascular occlusion upon deployment. The use of this new endovascular technology assures the operator that they have stopped blood flow at the treatment site. This creates an opportunity for shorter procedure times and less radiation exposure to patients and staff in addition to lower medical costs.”
ArtVentive CEO Jim Graham stated, “This is a testament to the hard work and dedication of our entire team. The EOS technology platform serves unmet clinical needs and provides a foundation for future innovation to expand into additional treatment areas. We look forward to sharing the technology with physicians in the U.S. and additional international markets as we commence our commercialization efforts.”
“We are excited to introduce the EOS device into the U.S. market,” said Dr. Leon Rudakov, president of ArtVentive. “We are expanding our case experience in Europe and anticipate further positive acceptance in the U.S. and global markets.”
Source: ArtVentive Medical Group Inc.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





