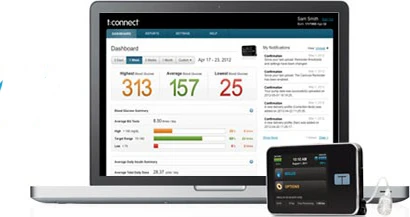
Tandem Diabetes Care Inc. officials announce that the company’s t:connect Diabetes Management Application, the web-based data management companion to the t:slim Insulin Pump, has been cleared for use by the U.S. Food and Drug Administration (FDA).
The t:connect Application is a Mac and PC-compatible data management software application that provides t:slim Pump users and their healthcare providers a fast, easy, and visual way to display data from the pump and supported blood glucose meters. This software empowers users to quickly and easily identify meaningful information and patterns, which allows them to fine-tune therapy and lifestyle choices for improved diabetes management.
The t:connect Application makes pump and meter data simple to understand with color-coded graphs and interactive reports. Data uploads from the t:slim Pump to the t:connect Application through a micro-USB connection without interrupting insulin delivery.
“We developed the t:connect Application with the same emphasis on ease of use employed in the development of the t:slim Insulin Pump,” says Kim Blickenstaff, president and CEO, Tandem. “The software was designed for speed and simplicity to encourage users to upload and review their data more frequently, and to improve the efficiency and productivity of visits with healthcare providers.”
All t:slim Pump users will be able to download the t:connect application from Tandem's website by the end of March 2013.
To learn more about the t:connect Diabetes Management Application, visit www.tandemdiabetes.com/tconnect.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





