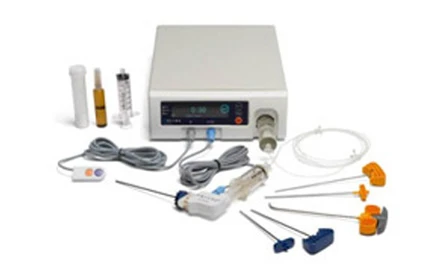
DFINE Inc., the developer of minimally invasive radiofrequency (RF) targeted therapies for the treatment of vertebral pathologies, today announced that it has received Medical Device License from Health Canada and the Secretaria de Salud (Ministry of Health) in Mexico to sell its StabiliT(R) Radiofrequency Targeted Vertebral Augmentation(TM) (RF-TVA) System throughout Canada and Mexico, respectively.
"StabiliT represents a significant advance in the treatment of osteoporotic compression fractures. The ability to target access and cement delivery while sparing valuable cancellous bone in patients who already have compromised bone structure due to osteoporosis is significant. I'm very pleased that we will now have access to this technology in Canada," says Kieran Murphy, M.D., deputy chief of radiology at University Health Network, Toronto, Canada.
According to Millennium Research Group, the global market for Minimally Invasive Vertebral Compression Fracture Treatments (MIVCFT) is poised for growth, due in large part to the aging global population and the large untreated population suffering from painful VCFs treated with ineffective conservative therapeutic measures.
"Approvals in Canada and Mexico are significant milestones for DFINE and the StabiliT system. With the North American market established, we will continue to execute our broader global expansion strategy, which will focus on potential growth markets including South America, Asia Pacific and the Middle East," says Kevin Mosher, chief executive officer of DFINE.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





