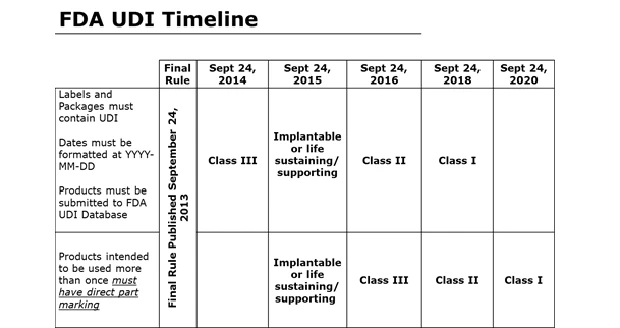
Washington, D.C. – The rush to compliance is in full swing. By Sept. 24, 2015, all implantable, life-saving or life-supporting devices must comply with the new UDI requirements. By 2018, all device makers must be in compliance. It's already clear that implementation and compliance with this new rule is a very big challenge.
You'll need to understand what UDI is, who it applies to, what the exceptions to the rule are, what deadlines you must meet, what UDI issuing agencies are, and how to work with them.
Implementing UDI is a huge challenge. That's why FDAnews developed the new management report Unique Device Identifier (UDI) Rule Implementation and Compliance Guide.
It walks you step-by-step through the key portions of the UDI final rule, providing you with all the implementation and compliance details you need to know.
With Unique Device Identifier (UDI) Rule Implementation and Compliance Guide, you'll gain a clear understanding of this complex new rule and learn to work with it more successfully. You will learn:
- The timetable for implementation
- Which devices must comply with the rule and which do not
- What information must be included on product labels
- How to submit device identification information to the GUDID
- About the accredited UDI issuing agencies and their roles
Unique Device Identifier (UDI) Rule Implementation and Compliance Guide is fully updated to reflect the final rule, chapter by chapter the report includes the critical information you need to get down to the real nitty gritty of complying with the UDI rule.
Source: FDANews
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





