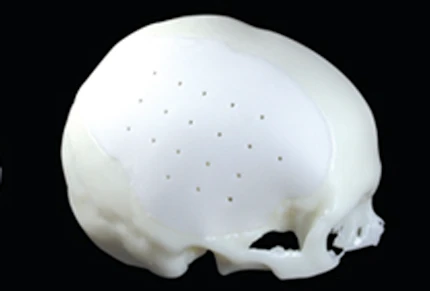
Kelyniam Global Inc., an emerging medical device manufacturing company that recently was granted FDA 510(k) approval, announced today that it has begun commercial shipment of its Custom Skull Implants (CSI). Kelyniam CSI's are seen by many in the medical community as a game-changer in the world of medical prosthetics.
Kelyniam Custom Skull Implants (CSI) are designed and manufactured for each individual patient to correct or replace bony voids in the cranial skeleton caused by trauma or birth defects. Streamlined CAD/CAM design and manufacturing techniques enable Kelyniam to deliver patient specific implants to surgeons in as little as 24 hours from the receipt of an order. The ability to provide custom implants quickly and accurately can reduce the time between trauma and implantation and provides surgeons additional treatment options.
"We are very excited to have begun commercial shipment of our state-of-the-art Custom Skull Implants," says James Ketner, president and CEO of Kelyniam. "We are confident our innovative technology, superior form, fit, and function will greatly enhance patient outcomes and empower surgeons by providing them with more and better treatment options than our competitors."
Kelyniam uses Invibio's PEEK-OPTIMA(R) for all of its custom implants.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





