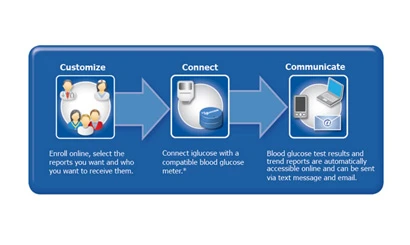
PositiveID Corporation has completed the development of its iglucose wireless communication system for real-time diabetes management. iglucose is a wireless device that connects to any data-capable glucometer to automatically communicate blood glucose readings to a secure online database. iglucose provides next generation, real-time data to improve diabetes management and help ensure patient compliance, data accuracy and insurance reimbursement. The Company expects to submit its application to the FDA for 510(k) clearance during the second quarter.
The iglucose system, a stand-alone, self-contained unit that operates without the use of a cell phone or personal computer, automatically queries data-capable glucometers for blood glucose readings and sends that data via wireless machine-to-machine (M2M) technology to an online database. At the patient’s discretion, blood glucose data can also be forwarded to physicians and caregivers at predetermined intervals.
Scott R. Silverman, chairman and CEO of PositiveID, says, “We are very pleased that in one year’s time, we have taken a bulky, bench-top device the size of a shoebox, and made it into a universal, wireless communication device the size of a smart phone that is ready to be submitted to the FDA for 510(k) clearance. We have lined up important marketing partners and believe that when commercialized, iglucose will fundamentally impact diabetes management by providing real-time glucose readings to loved ones, healthcare professionals and payers via wireless technology.”
According to a November 2009 study by researchers at the University of Chicago published in the journal Diabetes Care, the number of diabetics in the U.S., which currently stands at 23.7 million, may almost double in 25 years, and the annual cost of treating them may triple to $336 billion.
Latest from Today's Medical Developments
- Gore completes acquisition of Conformal Medical
- Medical textiles designed for cardiovascular, orthopedic, dental prosthetic applications
- Micro-precision 3D printing: Trends and breakthroughs in medical device manufacturing
- One-component, dual-cure adhesive system for medical device assembly
- #82 Manufacturing Matters - Forecasting 2026 with GIE Media's Manufacturing Group
- Flexing prosthetic finger offers lifelike appearance and movement
- How the fast-evolving defense market impacts suppliers
- Medtronic’s Hugo robotic-assisted surgery system makes US debut





