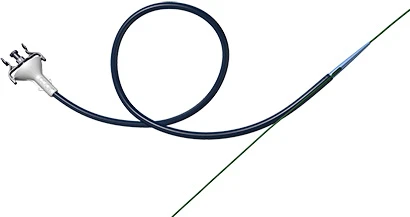
The Food and Drug Administration (FDA) approved Cook Medical's ureteral access sheath, featuring two options for placement. This approval now makes the product available in the United States. The Flexor Parallel Rapid Release Ureteral Access Sheath allows urologists to use a single wire guide that functions as both a working wire and a safety wire.
To place a ureteral access sheath, physicians will often use a working wire guide and a safety wire guide to allow continued access to the kidney. With the Flexor Parallel, physicians can place just one wire that guides the dilator and acts as a safety wire.
The Flexor Parallel has a slit on the dilator that guides the wire outside of the sheath. When the dilator is withdrawn, the sheath and the wire guide remain parallel to each other, which leaves a clear working channel through the sheath.
Physicians can also choose to place the Flexor Parallel in the same manner as a traditional Flexor sheath.
“The Flexor Parallel presents an opportunity to use less product and save costs for hospitals,” states Jean-Marc Creissel, global leader, Cook Medical’s Urology division. “This product means hospitals can stock one sheath that serves two placement preferences.”
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





