NinePoint Medical Inc. officials announce that it has received European CE mark approval for the NvisionVLE Imaging System. The CE Mark is for the company's "optical endomicroscopy imaging devices for diagnosis of mucosa and submucosa diseases." The company has also received ISO 13485:2003 certification, an international standard governing the requirements of a quality management system for medical devices and related services, for the company's Cambridge facility. The CE marking - or "Conformite Europeene" - certifies that a product has met European Union (EU) requirements for marketing in Europe.
"The ISO certification and CE mark approval are important milestones for NinePoint Medical and, along with our recently announced 510(k) clearance from the FDA, strongly position the NvisionVLE Imaging System for a commercial launch in 2013," states Charles Carignan, M.D., president and chief executive officer of NinePoint Medical.
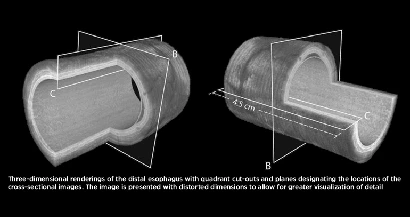
NinePoint Medical recently initiated a clinical trial to evaluate high-resolution optical imaging of Barrett's esophagus using the NvisionVLE Imaging System. Barrett's esophagus is one of the most common precursors to esophageal adenocarcinoma, the fastest growing cancer in the Western world. With the CE Mark, the company will now add two sites in Europe to the clinical trial. In addition, in January 2012 the company announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its NvisionVLE Imaging System for use as an imaging tool in the evaluation of human tissue microstructure by providing two-dimensional, cross sectional, real-time depth visualization. The NvisionVLE Imaging System is the first volumetric, optical coherence tomography (OCT) device cleared by the FDA for endoscopic imaging that generates cross sectional and longitudinal images simultaneously, in real time.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





