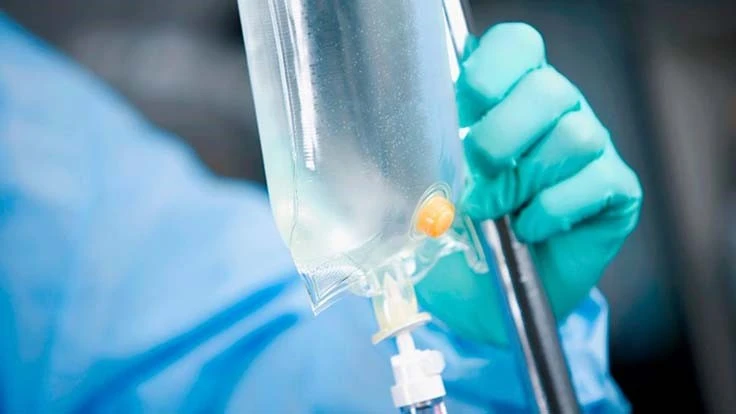
Wayne, Pennsylvania – According to officials from Colorite, a Tekni-Plex business unit specializing in custom medical-grade compounds, recent regulatory and market drivers, including cost pressures, are generating a material choice debate about polyvinyl chloride (PVC), thermoplastic elastomer (TPE) and rubber materials.
Many companies are trying to proactively address new regulatory dynamics – in the U.S. and globally – and pressure is being applied by healthcare systems that are already implementing strategic initiatives for phthalate-free patient environments. TPEs are being viewed as a replacement for PVC in applications where phthalate- or plasticizer-free materials are desired.
TPEs also are replacing thermoset rubbers (silicone, polyisoprene, and butyl rubber) used in elastomeric medical applications such as septa, stoppers, and syringe plungers. The drivers for rubber replacement are improved processing, cost effectiveness, and low extractables.
More recently TPEs also have been used to improve ergonomics, protection and/or function. TPEs are ideal for overmolding, which provides a softer touch and improved ergonomics (such as grip) for a variety of surgical tools and devices. This can improve instrument control and fatigue reduction during long procedures for medical professionals.
Colorite’s Cellene line of TPEs, suitable for a variety of uses in medical devices, are formulated to be silicone, latex, phthalate, halogen, and PVC-free using FDA-compliant raw materials to meet USP Class VI and ISO 10993 standards.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





