Apriso officials announced findings from a Cambashi research study. The study addressed the common challenges faced when medical device manufacturers seek to expand market share, quality and financial performance in the face of complex, dynamic regulatory environments. Those firms capable of confidently designing and executing their quality and other operational processes fared better, resulting in greater profits, efficiency, and operational performance.
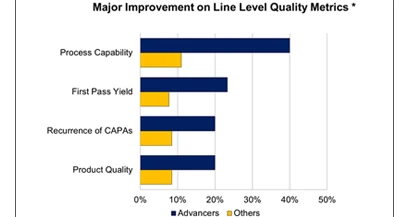
The report identified that the industry leaders or “Advancers” (top 25%) are able to increase product complexity, volume, quality, and profits – all at the same time. One of the key differentiators that separate the leaders from the rest is their process capability. Specifically, leaders define and execute production processes with superb precision. See the chart at right. For example, when examining a root cause analysis to determine why an out-of-conformance quality event occurred, industry leaders focus more effort on improving the process rather than trying to ascertain if the right processes were simply not executed properly.
In connection with the survey, Dave Empey, Director of Regulatory and Compliance at Zynex Medical, Inc., explains further: “With complaints, are you really getting to root cause, or just writing things up in a file in case the FDA shows up? There is a difference. If you are really trying to get to root cause and find out what you can do on the preventive side, it takes more time on the front end, but saves you pain and money down the road too.”
The report, sponsored by Apriso, focuses on the challenges medical device manufacturers face when seeking to grow, increase quality and improve financial performance in the face of complex, dynamic regulatory environments.
Key Findings:
- Industry leaders (top 25% performers) are increasing product complexity, volume, quality and profits at the same time, attributable in part by defining and executing production processes with superb execution
- A majority of the respondents anticipate launching at least 10% more new product introductions over the next three years; 24% of the respondents anticipate an increase of greater than 20%
- Industry leaders’ reliance upon IT systems interoperability is directly related to their success; better process integration across functions and plants leads to greater agility, quality, efficiency and profits
- While Quality by Design (QbD) is a great start, the benefits can’t be fully realized unless design is accompanied by what can be best referred to as “Quality by Execution;” the most efficient way to execute a Quality process is on an end-to-end basis, across IT systems, locations and functions
Rick Gallisa, life sciences industry director of Apriso, commented: “Medical device manufacturers are constantly challenged to design and manufacture innovative new products to remain viable. Product life cycles have shortened, global competition has intensified and greater regulatory oversight has put enormous pressure on operational efficiency.” Added Gallisa, “As the Cambashi research indicates, it is critical for medical device manufacturers to improve process and systems interoperability across all manufacturing operations and take a centralized approach to the global manufacturing process in order to best meet these significant challenges.”
Survey Methodology and Availability
The research for this study was conducted during the first half of 2012 and is based on 123 responses from medical device manufacturers. That survey was accompanied by a small set of telephone interviews. A copy of the research study is available free of charge on the Apriso website.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





