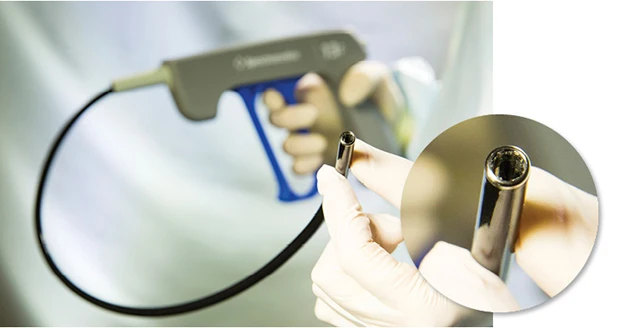
Colorado Springs, Colorado –The Spectranetics TightRail rotating dilator sheath platform has been designed for shaft flexibility and column strength to help physicians safely navigate the vasculature during cardiac lead extraction procedures.
The announcement includes TightRail and a companion product, TightRail mini rotating dilator sheath.
Spectranetics will now use the TightRail rotating dilator sheath platform to treat patients at facilities in Europe that are participating in the company's initial limited launch.
Spectranetics has designed the TightRail mini rotating dilator sheath to gain vascular access, dilating heavily fibrosed and calcified tissue.
TightRail features an advanced forward-facing dilating blade that remains shielded until activated. The tip feature rotates in both directions for efficient dilation. For procedural safety, the physician controls when the blade is exposed.
Following the completion of TightRail's first in-patient case, the company introduced the device at the Heart Rhythm Society's 35th Annual Scientific Sessions in San Francisco, US, earlier this month.
The TightRail rotating mechanical sheath platform obtained approval from the U.S. Food and Drug Administration (FDA) in April 2014.
Spectranetics lead management business unit sales and marketing senior vice-president Donna Ford-Serbu said that by providing a wider range of clinical solutions, the company is responding to a global need for innovative extraction options.
"Physicians are always looking for ways to serve their patient's needs, we are committed to providing tools that extend life and ensure physicians are successful at every turn," Ford-Serbu said.
In April, Spectranetics also received CE Mark and the FDA approvals for its SightRail manual dilator sheath.
The SightRail manual dilator sheath features visual indicators that show bevel orientation and tip alignment, supplementing fluoroscopy as a means to determine position and orientation.
The device features a longer inner sheath which gives physicians improved ability to grip and manipulate the device, especially when advancing and rotating.
Source: Spectranetics
Latest from Today's Medical Developments
- Gore completes acquisition of Conformal Medical
- Medical textiles designed for cardiovascular, orthopedic, dental prosthetic applications
- Micro-precision 3D printing: Trends and breakthroughs in medical device manufacturing
- One-component, dual-cure adhesive system for medical device assembly
- #82 Manufacturing Matters - Forecasting 2026 with GIE Media's Manufacturing Group
- Flexing prosthetic finger offers lifelike appearance and movement
- How the fast-evolving defense market impacts suppliers
- Medtronic’s Hugo robotic-assisted surgery system makes US debut





