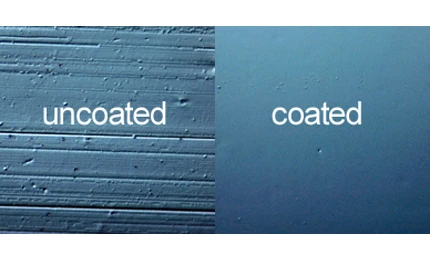
Officials from SurModics Inc., a leading provider of surface modification and in vitro diagnostic (IVD) technologies to the healthcare industry, announce that its proprietary hydrophilic technology is being used on Medtronic’s U.S. Food and Drug Administration (FDA) approved Resolute Integrity Drug-Eluting Stent (DES) delivery system for the treatment of coronary artery disease (CAD).
“We are extremely pleased that our hydrophilic coating was selected for use on the delivery catheter of Medtronic’s Resolute Integrity DES delivery system. SurModics values the long-standing relationship with Medtronic and remains steadfast in complementing the deliverability of the Resolute Integrity DES through our advanced hydrophilic coating platforms,” says Gary Maharaj, president and CEO of SurModics.
Advanced deliverability to navigate, successfully, the challenging anatomical sites faced by today’s interventional cardiologist is one unique attribute of the Resolute Integrity DES system.
Latest from Today's Medical Developments
- NextDent 300 MultiJet printer delivers a “Coming of Age for Digital Dentistry” at Evolution Dental Solutions
- Get recognized for bringing manufacturing back to North America
- Adaptive Coolant Flow improves energy efficiency
- VOLTAS opens coworking space for medical device manufacturers
- MEMS accelerometer for medical implants, wearables
- The compact, complex capabilities of photochemical etching
- Moticont introduces compact, linear voice coil motor
- Manufacturing technology orders reach record high in December 2025





